
[ad_1]
It may start with a feeling of lack of energy, or you may feel a little irritable. You may have a headache or trouble concentrating. Your brain is sending you a message: you are hungry, look for food. Studies in mice have identified a group of cells called AgRP neurons, located near the bottom of the brain, that can trigger this unpleasant feeling of hunger. They are close to the blood supply to the brain, giving them access to hormones from the stomach and fat tissue that signal energy levels. When energy is low, they act on other brain areas to promote eating.
By eavesdropping on AgRP neurons in mice, scientists have begun to unravel how these cells activate and encourage the animals to forage when they are low on nutrients, and how they sense the arrival of food in the gut only to deactivate again. The researchers have also found that the activity of AgRP neurons is altered in mice with anorexia-like symptoms, and that activation of these neurons may help restore normal eating patterns in these animals.
Understanding and manipulating AgRP neurons could lead to new treatments for both anorexia and overeating. “If we could control this feeling of hunger, we could better control our diets,” says Amber Alhadeff, a neuroscientist at the Monell Chemical Senses Center in Philadelphia.
AgRP neurons seem to play a key role in appetite: by turning them off in adult mice, the animals stop eating and may even starve. Conversely, if the researchers fire the neurons, the mice jump onto their plates and gorge themselves on food.
Experiments carried out in several laboratories in 2015 had already helped to illustrate what AgRP neurons do. The researchers found that when the mice had not eaten enough, the AgRP neurons fired more frequently. But all it took was the sight or smell of food (especially something delicious, like peanut butter or chocolate) for this activity to subside in a matter of seconds. The scientists concluded that AgRP neurons make animals search for food. Having found the food, they stop shooting with the same intensity.
A research team, led by neuroscientist Scott Sternson of the Janelia Research Campus in Ashburn, Virginia, also showed that the activity of AgRP neurons appears to make mice feel bad. To demonstrate this, the scientists genetically engineered mice so that AgRP neurons would start firing when an optical fiber was illuminated into their brains (the fiber still allowed the mice to move freely). They placed these modified mice in a box with two distinct areas: one black with a plastic mesh floor and one white with a soft tissue paper floor. If the researchers activated the AgRP neurons every time the mice entered one of the two zones, the mice began to avoid that region.
Sternson, who now works at the University of California, San Diego, concluded that AgRP activation was “slightly unpleasant.” That makes sense in the wild, he says: Every time a mouse leaves its nest, it risks being attacked by predators, but it must overcome that fear to forage and feed. “These AgRP neurons are kind of a nudge so that, in a dangerous environment, you go out looking for food to stay alive.”
Sternson’s 2015 study had shown that although the sight or smell of food calms AgRP neurons, it’s only temporary: Activity spikes again if the mouse can’t go ahead and eat the snack. Through additional experiments, Alhadeff and his colleagues discovered that AgRP neurons are most reliably turned off by calories reaching the gut.
First, Alhadeff’s team gave the mice a calorie-free treat: a gel with artificial sweetener. When the mice ate the gel, the activity of the AgRP neurons decreased, as expected, but only temporarily. As the mice learned that they couldn’t get any nutrients from this snack, their AgRP neurons became less and less responsive to each bite. So, as the animals learn whether a treat actually nourishes them, the neurons adjust the hunger dial accordingly.
Next, the team used a catheter implanted through the abdomen to deliver calories, in the form of Ensure nutrition drink, directly to the stomach. In this way, any sensory signal that the food was about to arrive was avoided. The result was a longer decline in AgRP activity. In other words, it’s the nutrients in food that turn off AgRP neurons for a long time after a meal, Alhadeff concluded.
Since then, Alhadeff has begun to decipher the messages that the stomach sends to AgRP neurons, and has discovered that they are nutrient-dependent. Fat in the intestine activates a signal through the vagus nerve, which runs from the digestive tract to the brain. Simple sugar (glucose) sends a signal to the brain through the nerves in the spinal cord.
His team is now investigating why these multiple pathways exist. He hopes that by better understanding how AgRP neurons drive foraging, scientists may be able to find ways to help keep people from gaining weight. Although scientists and dieters have been searching for such treatments for over a century, easy, safe, and effective treatments have been difficult to find. Thus, the latest weight-loss drugs, such as Wegovy, partly act on AgRP neurons, but have unpleasant side effects, such as nausea and diarrhea.
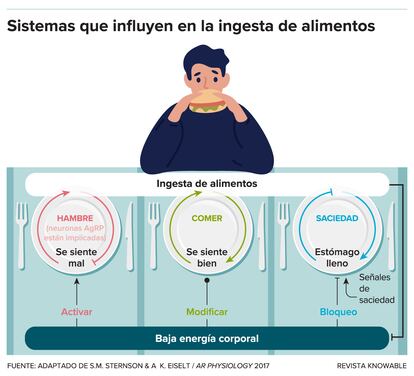
Therapies targeting AgRP neurons alone probably wouldn’t completely solve the weight problem, because food seeking is only one component of appetite control, says Sternson, who reviewed the major appetite controllers in the Annual Review of Physiology in 2017. Other brain areas, which sense satiety and make high-calorie foods pleasurable, also play important roles, the neuroscientist adds. That’s why, for example, you eat that piece of pumpkin pie at the end of the Thanksgiving meal, even though it’s already loaded with turkey and mashed potatoes.
overcome anorexia
The flip side of overeating is anorexia, and here too the researchers believe that investigating AgRP neurons could lead to new treatment strategies. People with anorexia avoid food, to the point of losing weight dangerously. “Eating is really aversive,” says Ames Sutton Hickey, a neuroscientist at Temple University in Philadelphia. There is no specific medication for anorexia; Treatment may include psychotherapy, general medications such as antidepressants and, in the most severe cases, force-feeding through a tube inserted through the nose. People with anorexia are also often restless or hyperactive and may exercise excessively.
Researchers can study this condition using a mouse model of the disease known as activity-based anorexia, or ABA. When scientists limit the food available to mice and provide them with a wheel to run on, some of them enter a state similar to anorexia, eating less than what is offered and running on the wheel even during the day, when mice are normally inactive. “Something very addictive happens to these animals,” says Tamas Horvath, a neuroscientist at Yale School of Medicine. “Basically, it turns them on not eating and exercising.”
It is not a perfect model for anorexia. Mice presumably don’t face any of the social pressures to stay thin that humans do; For their part, people with anorexia usually have no limits on their access to food. But it’s one of the best anorexia mimics around, says Alhadeff: “I think it’s the best we’ve got.”
To find out how AgRP neurons might be involved in anorexia, Sutton Hickey carefully monitored the ABA mice’s food intake. She compared them to mice that were given a restricted diet, but had a locked exercise wheel and did not develop ABA. She discovered that the ABA mice ate less than the others. And when they ate, their AgRP activity didn’t drop as it should after filling their stomachs. Something was wrong with the way neurons responded to hunger and food signals.
Sutton Hickey also found that he could fix the problem when he engineered ABA mice so that AgRP neurons would fire when the researchers injected them with a certain chemical. These mice, when treated with the chemical, ate more and gained weight. “That speaks volumes about the importance of these neurons,” says Horvath, who was not involved in the work. “It shows that these neurons are the good ones, not the bad ones.”
Sutton Hickey says the next step is to find out why AgRP neurons respond abnormally in ABA mice. She hopes there may be some key molecule to target for a drug to help people with anorexia.
Taken together, the work on AgRP neurons is giving scientists a much better picture of why we eat when we do, as well as new leads, perhaps, for drugs that could help people turn disordered eating into healthy habits.
Article translated by debbie ponchner.
This article originally appeared on Knowable in Spanisha non-profit publication dedicated to making scientific knowledge available to everyone.
You can follow THE COUNTRY Health and Well-being in Facebook, Twitter and instagram.
[ad_2]

